The Rise of The Disappearing Polymorphs [Hackaday]
Science and engineering usually create consistent results. Generally, when you figure out how to make something, you can repeat that at will to make more of something. But what if, one day, you ran the same process, and got different results? You double-checked, and triple-checked, and you kept ending up with a different end product instead?
Perhaps it wasn’t the process that changed, but the environment? Or physics itself? Enter the scary world of disappearing polymorphs.
Point of No Return
Imagine you’re working at a laboratory that creates pharmaceuticals. You figure out the reactants and the chemistry involved to make a new drug. You design a production line, and your new factory starts churning out the drug in mass quantities. This goes well for years, until suddenly, the drugs stop working.
You run an analysis, and the drugs coming out of the factory aren’t what you designed at all. They’re a weird new form of the same chemical with a different crystal structure, and they’re no longer working the same way. What happened?
You’ve probably come across the case of a disappearing polymorph. This is where the original version of a chemical’s crystal structure becomes impractical or impossible to produce. Instead, you tend to end up with a new version instead, typically a more stable, lower-energy version. The mere presence of this newer, more stable version tends to convert the original version into the new form quite easily. Since the new form is more stable, it tends to become difficult to convert the product back to the original form, or functionally, to produce it at all.
Paroxetine Problems
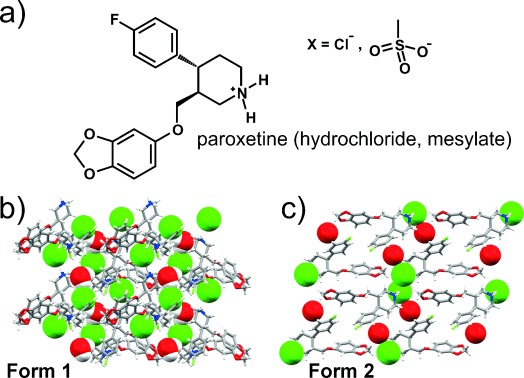
A great case study exists in paroxetine hydrochloride, an SSRI medication. The initial form of the drug was developed in the 1970s, and was known as paroxetine anhydrate. It was produced as a hygroscopic, chalky powder. However, in 1984, a new version spontaneously popped up at sites in the UK that were scaling up production. The new ‘hemihydrate’ crystal form was more stable. Drug in the anhydrate form would spontaneously convert into hemihydrate whenever the two came into contact in the presence of water or mere humidity.
The issue caused legal problems down the line. Years later, other drug manufacturers wished to produce paroxetine, too. The patent on paroxetine anhydrate had ran out, so generic manufacturer Apotex moved to begin production. The issue was that the company found it could not produce the original form. Instead, its product inevitably came out as paroxetine hemihydrate. It’s believed that the Earth’s atmosphere had functionally become populated by trace amounts of paroxetine hemihydrate, to the point where any paroxetine anhydrate would immediately be transformed into the new structure.
By this time, GSK was the company that held a still-active patent on paroxetine hemihydrate. It sued Apotex, arguing that its generic pills contained paroxetine hemihydrate that had been created through the atmospheric seeding process. The courts accepted GSK’s submission on this point, but ruled in favor of Apotex’s right to continue producing its generic pills. It was noted Apotex could not be held responsible for the issue of uncontrolled crystal seeding.
Later research saw two separate companies independently create another polymorph. Both Synthon and SmithKline Beecham sought patents for the production of polymorphs known as paroxetine mesylate. However, a similar problem cropped up shortly after. Any attempt to create the Synthon polymorph would end up creating the Beecham structure instead. This lead to much confusion over whether Synthon’s version was a new case of a disappearing polymorph, or whether the company had made errors in its patent work. Ultimately, no satisfying consensus was reached as to the truth of the matter.
Treatment Failure
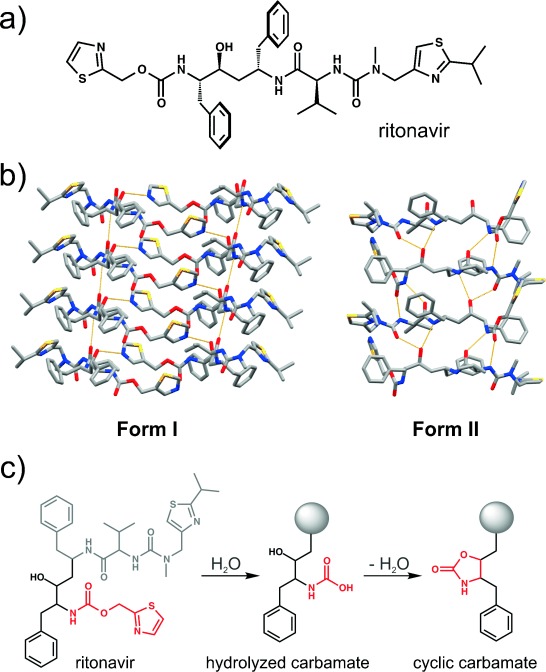
Sadly, disappearing polymorphs can create more than legal woes. Ritonavir was released for public use in 1996, a crucial antiretroviral drug used in the fight against HIV. In its original crystal form, known as ‘Form I”, it was quite soluble and medically useful for treating the condition. However, in 1998, “Form II” was discovered. This was a more stable polymorph that existed at a lower energy level. The problem was that this crystal form was much less soluble. This made the drug less bioavailable, ruining its effectiveness at treating the disease.
The existence of Form II threatened the production of the useful form of the drug. Any laboratory that saw the introduction of Form II was unable to produce Form I afterwards. It was speculated by researchers that individuals that had worked in such labs could carry traces of the new form, and potentially poison facilities that were still producing Form I. In the space of a few weeks, everywhere that could once produce Form I was rapidly turning out only Form II instead.
Due to problems with production and the lack of efficacy of Form II ritonavir, the drug was pulled from the market. This lead to thousands of patients going without medication for their condition, and losses of over $250 million for the manufacturer, Abbott. The company held press conferences that highlighted the gravity of the issue.
“This is why all of us at Abbott have been working extremely hard throughout the summer [of 1998], often around the clock, and sometimes never going home at night. We have been here seven days a week and we will continue to do so. We have cancelled vacations and asked our families for their understanding and support. This is not an issue that we take lightly.”
Eventually, the problem was overcome. Researchers found a way to produce Form I under highly controlled conditions. The product had to be sold in a special refrigerated gel cap, compared to its original delivery form of a non-refrigerated capsule. Later developments included a combination of lopinavir and ritonavir that did not require lower temperatures to remain stable, and a new form of melt-extruded Form I ritonavir tablet that hit the market in 2010.
What Can Be Done
Scientists are some what at the mercy of nature when it comes to disappearing polymorphs. New polymorphs can pop up without warning, while tiny seed crystals can quickly contaminate entire labs, factories, and indeed, the world. There’s little defence. The only solution is doing hard chemistry—either to find ways to make original polymorphs survive the new world, or to find other new polymorphs that are still useful and still producible.
Ultimately, though, new polymorphs can be a pharmaceutical engineer’s nightmare. They can ruin a drug and ruin a factory overnight. Stories of ritonavir and other drugs will remain cautionary tales for this very reason.

![the-rise-of-the-disappearing-polymorphs-[hackaday]](https://i0.wp.com/upmytech.com/wp-content/uploads/2024/07/198138-the-rise-of-the-disappearing-polymorphs-hackaday-scaled.jpg?resize=800%2C445&ssl=1)