MIT Engineers Pioneer Cost-Effective Protein Purification For Cheaper Drugs [Hackaday]
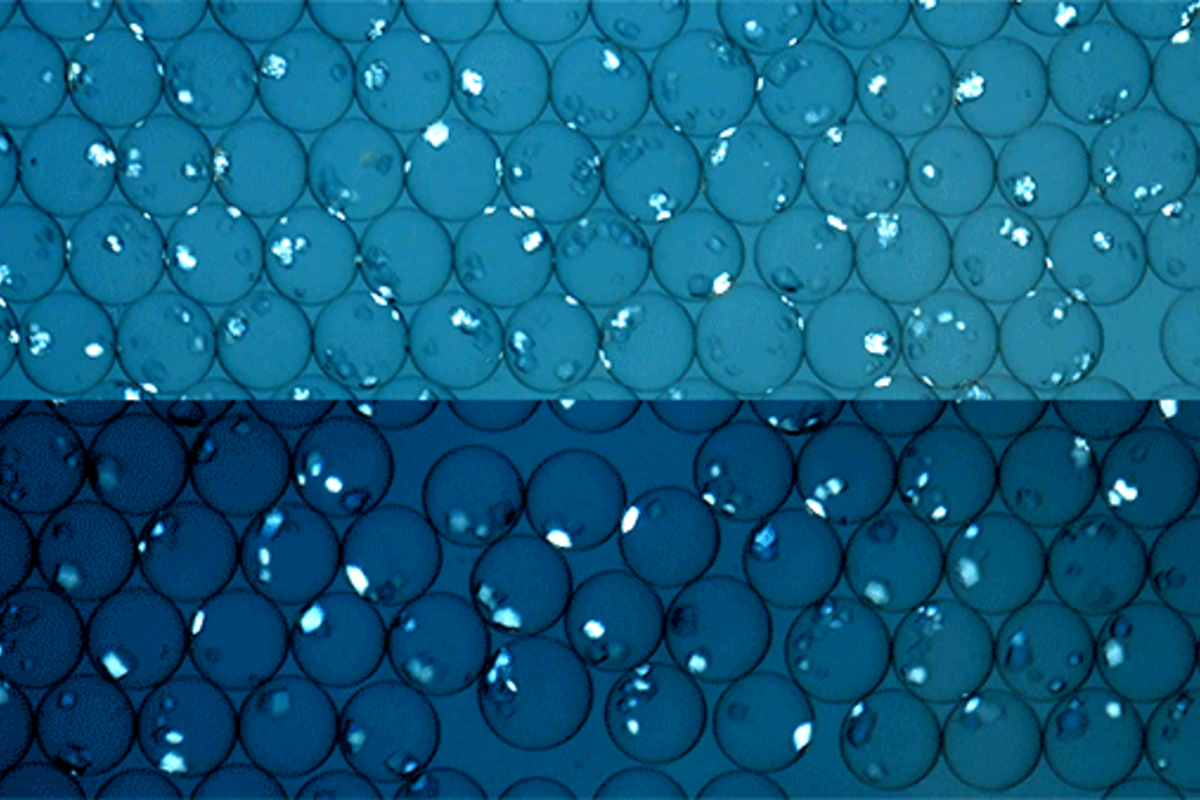
There are a wide variety of protein-based drugs that are used to treat various serious conditions. Insulin is perhaps the most well-known example, which is used for life-saving treatments for diabetes. New antibody treatments also fall into this category, as do various vaccines.
A significant cost element in the production of these treatments is the purification step, wherein the desired protein is separated from the contents of the bioreactor it was produced in. A new nanotech discovery from MIT could revolutionize this area, making these drugs cheaper and easier to produce.
The Old Ways

Traditional protein purification relies heavily on chromatography, a method that separates proteins based on their size. The need for specialized materials in chromatography, however, substantially drives up the cost of manufacturing.
Chromatography, at its core, is a physical separation technique that separates components between two phases: the stationary phase (a solid or a liquid supported on a solid) and the mobile phase (a liquid or a gas). In the context of protein purification for drug manufacture, chromatography typically takes advantage of differences in properties like protein size, charge, or binding affinity to selectively separate the target protein from other biological material present in the bioreactor. The protein mixture is passed through a column packed with the stationary phase, and different proteins interact with the stationary phase to different extents, thus moving at varying speeds and emerging from the column at different times. By this method, the desired protein can be purified.
The complexity and high cost associated with chromatography largely result from the need for specialized materials and equipment. For instance, the stationary phase materials used in the columns can be incredibly expensive, especially for size-exclusion or affinity chromatography. Additionally, chromatography often requires multiple rounds to achieve high purity levels, meaning more time, materials, and energy are consumed. Lastly, the process requires meticulous control over conditions like pH and temperature, contributing to the difficulty and expense involved. The culmination of these factors results in chromatography being one of the most cost-intensive steps in the production of protein drugs.
We’re Like Crystal
Rather than tweaking the conventional method, the MIT research team, led by mechanical engineering Professor Kripa Varanasi, explored an alternative: protein crystallization. By assembling proteins into large regular crystals, it’s possible to get very pure samples of the protein without external contaminants. The technique is typically considered too slow for industrial purposes. It’s also ineffective at low protein concentrations, and in “dirty” solutions filled with a mixture of different biological materials. The MIT team innovated a solution to enhance the process’s efficacy and solve these problems through the use of specialized nanoparticles.
In a study published in ACS Applied Materials and Interfaces, the MIT team reported the successful use of gold nanoparticles to facilitate the rapid crystallization of proteins. The nanoparticles were coated with bioconjugates, which are materials designed to help different molecules form links together. When exposed to these nanoparticles, coated with maleimide and NHS bioconjugates, protein molecules binded to the surface bioconjugates, aligning in a specific orientation that kicked off further protein crystal formation.
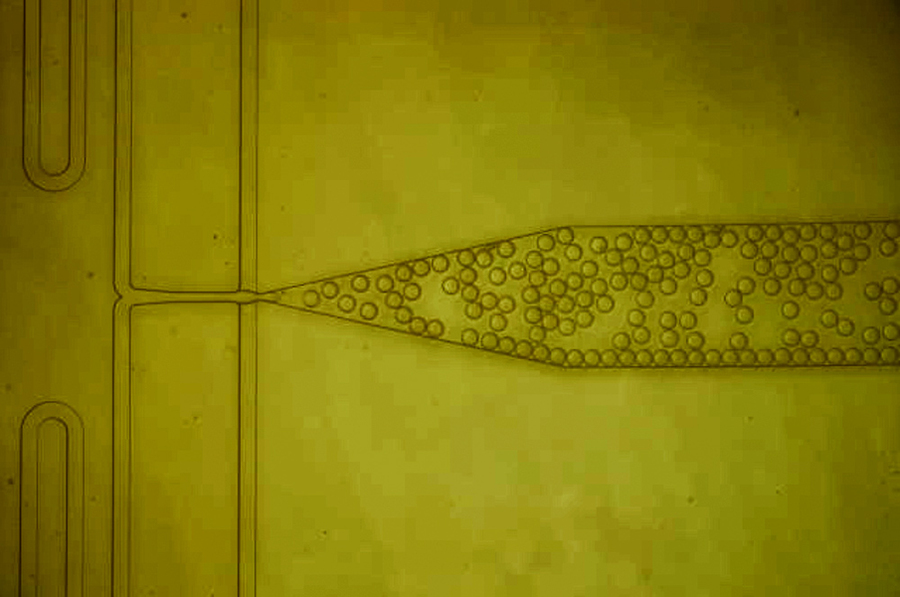
The team demonstrated this approach with lysozyme and insulin, with results suggesting that it could also be applied to a wide range of proteins, including antibody drugs and vaccines. Furthermore, the engineered nanoparticles significantly reduced the time period until crystal formation begins, while also increasing the speed of crystal growth.
The team also pioneered modern techniques to speed their research. The researchers employed machine learning to analyze the data gathered from thousands of crystal images. The use of ML tools allowed them to efficiently process large datasets, identifying when crystals formed in each image without the need for time-consuming manual counts. The lowly grad students that would normally be tasked with this grunt work may celebrate this development.
The research has the potential to make biologic drugs more accessible in developing countries. The MIT team is currently focused on scaling the process for industrial use and proving its efficacy with other proteins, like monoclonal antibodies and vaccines.
While it’s clear that further research and development are necessary, this pioneering approach could be a game-changer in the world of protein drug manufacturing. Using nanotechnology to create a more cost-effective and efficient method for protein purification could make protein-based medication far more accessible globally. That can only be a good thing!

![mit-engineers-pioneer-cost-effective-protein-purification-for-cheaper-drugs-[hackaday]](https://i0.wp.com/upmytech.com/wp-content/uploads/2023/06/130169-mit-engineers-pioneer-cost-effective-protein-purification-for-cheaper-drugs-hackaday.png?resize=800%2C445&ssl=1)