Inverse Vaccines Could Help Treat Autoimmune Conditions [Hackaday]

Autoimmune diseases occur when the immune system starts attacking the body’s own cells. They can cause a wide range of deleterious symptoms that greatly reduce a patient’s quality of life. Treatments often involve globally suppressing the immune system, which can lead to a host of undesirable side effects.
However, researchers at the University of Chicago might have found a workaround by tapping into the body’s own control mechanisms. It may be possible to hack the immune system and change its targeting without disabling it entirely. The new technique of creating “inverse vaccines” could revolutionize the treatment of autoimmune conditions.
Identification: Friend or Foe
The body’s immune response is normally a good thing, defending us from various pathogens. Indeed, it’s crucial to our survival; individuals with suppressed immune systems must take all kinds of precautions to avoid harm on a regular basis. In autoimmune diseases, though, the body’s immune response gets misdirected, attacking the body’s own cells. Suppressing the entire immune system can solve the autoimmune problem, but leaves the body with precious little defence against actual pathogens.
A new method of molecular hacking may be key to solving this problem, according to a new paper published in Nature. The idea is simple: it aims to reprogram the immune system, instructing it ignore certain targets that it shouldn’t be attacking. It’s basically the opposite of how a vaccine works, hence the name “inverse vaccine.” They’re intended to advise the immune system on what not to kill.
The immune system determines what to attack by focusing on antigens. These are molecular markers, like proteins or strings of amino acids, that the immune system can recognize. For example, the COVID-19 coronavirus features prominent spike proteins. When presented with these spikes, our immune system typically identifies them as foreign and unfriendly, and scrambles to attack them. This happens more quickly once we’ve had a vaccine or previous exposure to the virus, as our body remembers how to recognise those antigens. The problem is when our immune system gets confused, and starts identifying our own cells as targets for attack. In many autoimmune diseases, it’s unclear why the body begins to attack itself, but research continues into theories around genetic predispositions, environmental factors, and responses to viral and bacterial infections.
To try and reprogram the immune system, scientists developed a polymer that was glycosylated with N-acetylgalactosamine, known as pGal for short. Attached to this polymer is the antigen causing the immune response, connected via a “self-immolative linker.” Once the polymer is ingested by an immune system cell in a process called endocytosis, this linker breaks away, releasing the antigen inside the cell’s regulatory environment. Once this happens, the immune system ceases to treat the antigen as a threat.
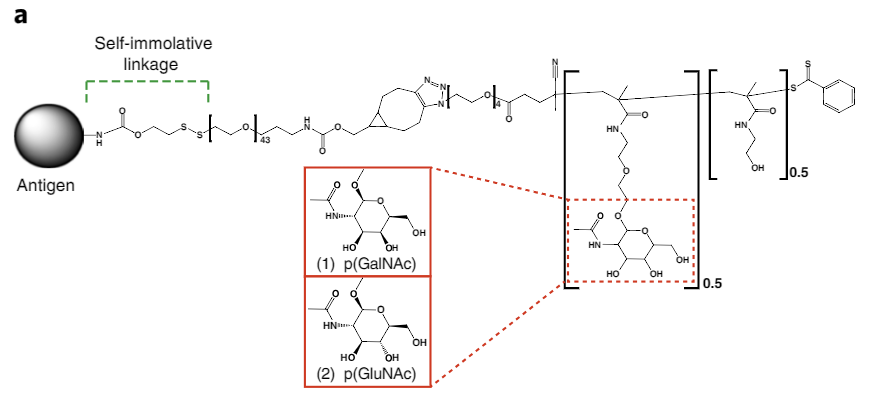
The idea for this method came from the body itself. Normally, when a cell dies naturally, the immune system does not overreact. This is because the liver uses pGal as a tag for molecules from dying cells, which tells the immune system to ignore these molecules. The researchers simply leveraged this existing mechanism as a way to get the immune system to ignore other targets as desired.
Early testing showed that the technique could be used to prevent Type 1 diabetes from occurring in a mouse model by preventing the autoimmune response to insulin-producing cells. Later work showed that the technique could also potentially be used to treat autoimmune diseases that had already occurred. This was achieved by demonstrating that an immune system could be programmed to stop attacking the myelin coating on nerves in animals suffering a multiple sclerosis-like disease. The nerves regained function in time, and the animal’s symptoms were reversed.
Lead author of the study, Jeffrey Hubbell, is buoyant about the possibilities. “In the past, we showed that we could use this approach to prevent autoimmunity,” said Hubbell, adding “But what is so exciting about this work is that we have shown that we can treat diseases like multiple sclerosis after there is already ongoing inflammation, which is more useful in a real-world context.”
Phase I safety trials of an antigen therapy based on this work have taken place with patients suffering from celiac disease, along with a further Phase I safety trial for treating multiple sclerosis. As of yet, no “inverse vaccines” are yet approved for clinical use, but there is strong potential for this technique to improve our ability to treat and manage autoimmune conditions.

![inverse-vaccines-could-help-treat-autoimmune-conditions-[hackaday]](https://i0.wp.com/upmytech.com/wp-content/uploads/2023/09/143971-inverse-vaccines-could-help-treat-autoimmune-conditions-hackaday-scaled.jpg?resize=800%2C445&ssl=1)