Crab Shells Massively Improve Zinc-Ion Batteries [Hackaday]

In the fast-moving world of battery research, scientists are constantly on the lookout for innovative materials with the right properties to help improve energy storage. Meanwhile, batteries are in greater demand than ever as production of EVs and renewable energy projects ramp up to new heights.
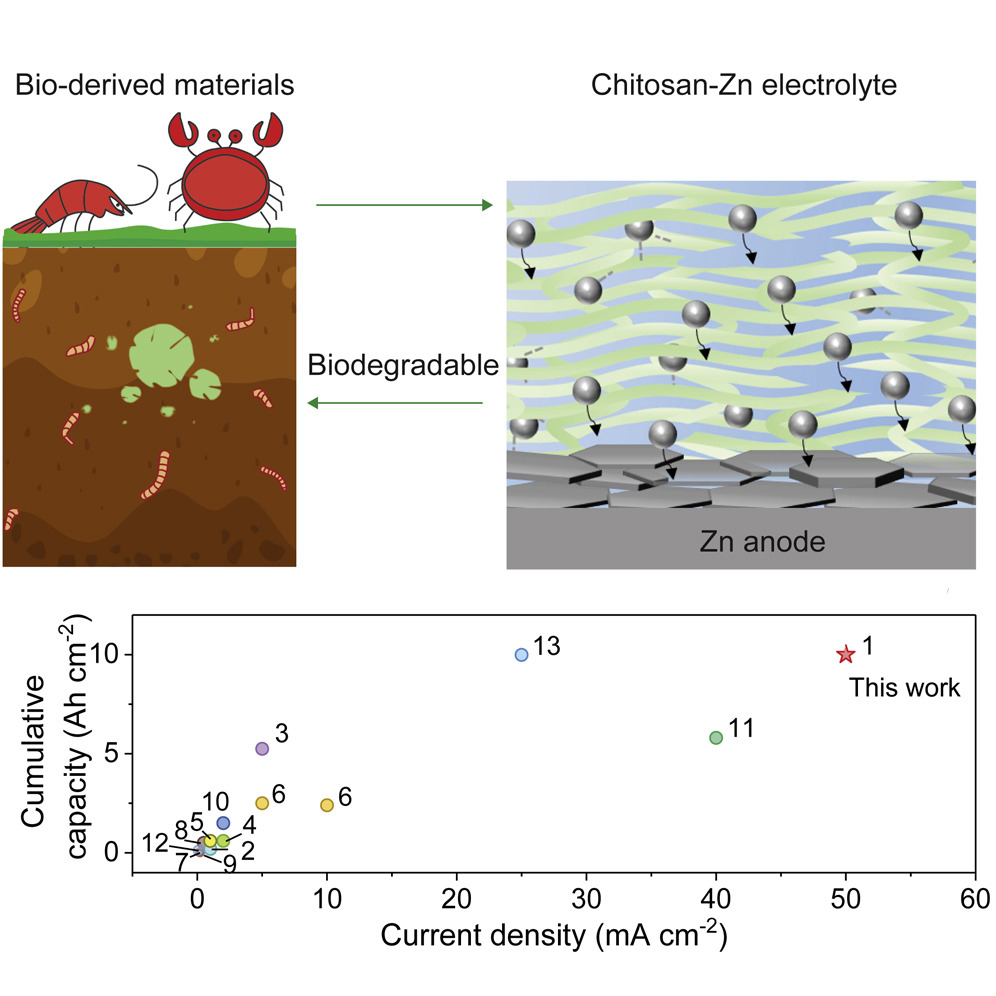
In the hunt for new and better battery materials, scientists found an unexpected hero: crab shells.Researchers at the University of Maryland have uncovered a remarkable breakthrough by exploring their use in battery production.
Rock Lobster

The quest for advanced energy storage materials has been a driving force behind numerous research endeavors worldwide. Traditional lithium-ion batteries have come to dominate in many fields. They power the vast majority of our personal electronic devices, as well as electric vehicles and large grid storage projects. While effective, face limitations in terms of cost, finite resource availability, and safety concerns. As a result, scientists have been exploring alternative battery technologies that offer improved performance, longevity, and scalability.
One such alternative gaining traction is zinc-ion batteries, which have shown promise due to their lower cost and abundance of raw materials. They have comparable performance in some respects, though do have lower power output which makes them suited for less-demanding storage roles. Thus far, their practicality has been hindered by a limited lifespan, hampering their viability. However, the innovative use of material from crab shells could change all that.
The material in question is chitosan—a substance found in abundance within crab and lobster shells. Scientists believe it could be used as a key ingredient in zinc-ion batteries. It’s an unconventional approach that showcases the versatility of natural resources, and could hold the potential to revolutionize the energy storage landscape.
The researchers at the University of Maryland sought to harness the properties of chitosan—a carbohydrate found abundantly in the hard outer skeletons of shellfish—for energy storage applications. By incorporating chitosan into the gel membrane of zinc-ion batteries, they achieved unprecedented results, according to the research paper. The batteries exhibited an impressive year-long lifespan in cycle testing, retaining 70% of their initial capacity—an astonishing improvement compared to existing zinc-ion battery technology.
Usually, zinc-ion batteries suffer from zinc metal dendrites, which disable the battery after a relatively low number of cycles. This is largely down to the aqueous electrolytes typically used, which allows zinc to form dendritic spikes due to inhomogenous deposition of the metal on the anode over time.
When chitosan is used as part of the electrolyte of the battery, it brings several benefits to the table. It offers strong conductivity and good mechanical strength, and massively improves the cycle stability of zinc-ion batteries. It achieves this by changing the way zinc is deposited on the anode surface. When using the chitosan-based electrolyte, the zinc forms hexagonal platelets that sit neatly in parallel on the anode, instead of forming large dendrites that pierce the battery separator. As a further benefit, the chitosan electrolyte is able to achieve this without unduly compromising the conductivity of the battery. In testing, the batteries were shown to still be stable after over 400 cycles at a 2C discharge rate, while also demonstrating the ability to charge at up to 20C.
Furthermore, the inclusion of chitosan mitigates safety concerns often associated with lithium-ion batteries. The use of this non-flammable electrolyte reduces the risk of explosions and fire hazards, paving the way for safer and more sustainable energy storage solutions. A further bonus is that zinc-ion batteries could be fabricated in a sustainable and biodegradable manner using chitosan-based electrolyte.
The Glory of Byproducts
Historically, crab shells are discarded from the crab meat production process, and have found limited utility in applications such as fertilizer and animal feed. However, the emergence of chitosan-based batteries presents an exciting opportunity to transform these shells into a valuable resource for renewable energy storage. Mass chitosan production could yet become a serious side-hustle for big players in the seafood industry.
However, while the research has attracted considerable attention, several challenges remain on the path to commercialization. The process of refining and scaling up the production of chitosan-based batteries requires further development, investment, and collaboration with industry partners.
The unexpected alliance between battery research and crab shells exemplifies the remarkable potential of natural materials. By leveraging chitosan from discarded crab shells, scientists have achieved breakthroughs in energy storage that could transform the renewable energy landscape. As the world continues to hunt for new battery solutions, the humble crab could be our latest ally in this area.

![crab-shells-massively-improve-zinc-ion-batteries-[hackaday]](https://i0.wp.com/upmytech.com/wp-content/uploads/2023/07/133463-crab-shells-massively-improve-zinc-ion-batteries-hackaday-scaled.jpg?resize=800%2C445&ssl=1)