Another Room-Temperature Superconductivity Claim and Questions of Scientific Integrity [Hackaday]

In early March of 2023, a paper was published in Nature, with the researchers claiming that they had observed superconductivity at room temperature in a conductive alloy, at near-ambient pressure. While normally this would be cause for excitement, what mars this occasion is that this is not the first time that such claims have been made by these same researchers. Last year their previous paper in Nature on the topic was retracted after numerous issues were raised by other researchers regarding their data and the interpretation of this that led them to conclude that they had observed superconductivity.
According to an interview with one of the lead authors at the University of Rochester – Ranga Dias – the retracted paper has since been revised to incorporate the received feedback, with the research team purportedly having invited colleagues to vet their data and experimental setup. Of note, the newly released paper reports improvements over the previous results by requiring even lower pressures.
Depending on one’s perspective, this may either seem incredibly suspicious, or merely a sign that the scientific peer review system is working as it should. For the lay person this does however make it rather hard to answer the simple question of whether room-temperature superconductors are right around the corner. What does this effectively mean?
Cold Fusion
What room-temperature super conducting materials and cold fusion have in common is that both promise a transformative technology which would alter the very fabric of society. From near-infinite, cheap energy, to zero loss transmission lines and plummeting costs of MRI scanners and every other device that relies on superconducting technology to work, either technology by itself would cause a revolution. At the same time, as with any such revolutionary new technology, it would also stand to make a select number of people very wealthy.
At this point in time, the term ‘cold fusion’ has become synonymous with ‘snake oil’, with researchers finding themselves unable to replicate the results of the original 1989 experiment by Martin Fleischmann and Stanley Pons. This experiment involved infusing a palladium electrode with deuterons (nucleus of deuterium), from surrounding heavy water (D2O), before running a current through the electrodes.
After the media backlash from the failure to replicate these results poisoned the very subject of ‘cold fusion’, its researchers have since maintained a low profile, calling the research area that of ‘low energy nuclear reactions’ (LENR) and generally shied away from the public eye. What is tragic about LENR is that at its core it’s essentially lattice confinement fusion (LCF), which NASA researchers at Glenn Research Center recently demonstrated in a study using erbium to provide the metal lattice.
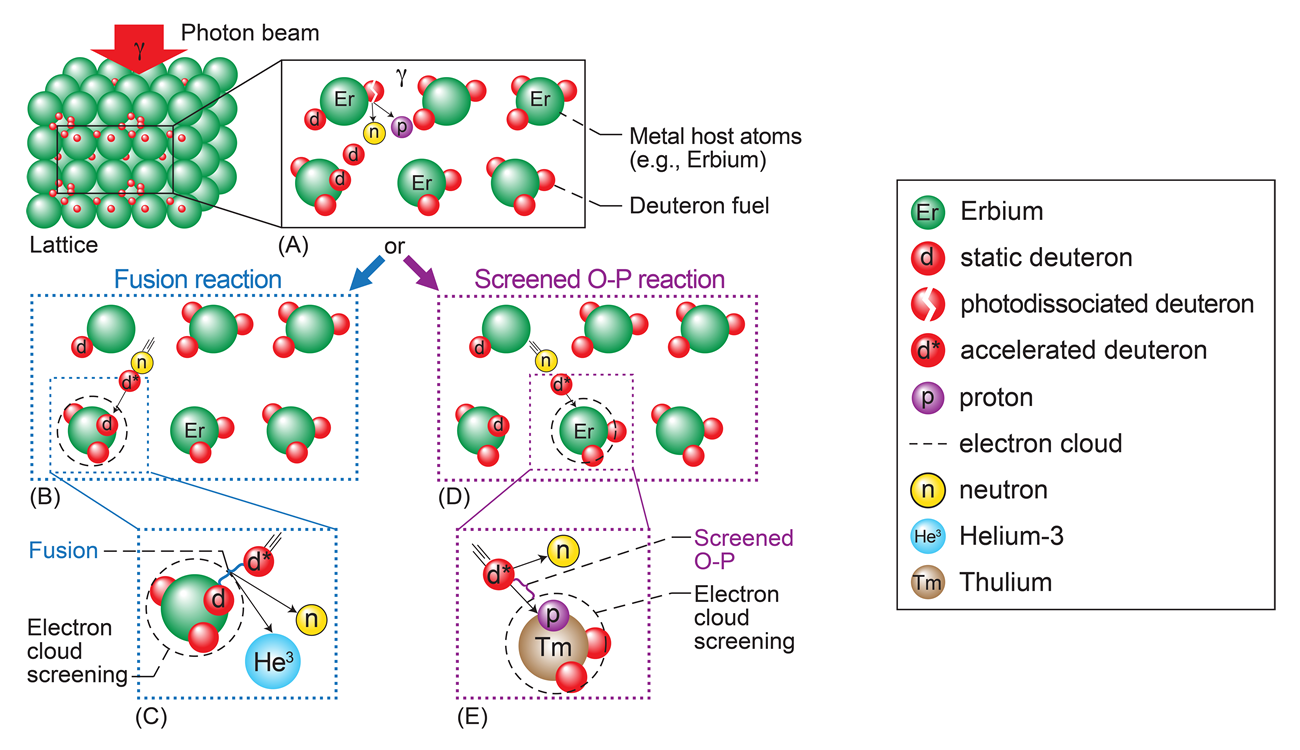
The NASA LCF experimental setup involves a similar loading of the metal with deuterons as in the Fleischmann-Pons experiment. The advantage of LCF over plasma-based fusion approaches as performed in tokamaks is that inside the host metal lattice the distance between the deuteron nuclei is less than that of D-T fuel nuclei in deuterium/tritium fuel plasma, making theoretically overcoming the Coulomb barrier and initiating fusion easier while not requiring high pressures or temperatures.
To initiate D-D fusion, the NASA researchers used 2.9+ MeV gamma beams to irradiate the deuterons, with the researchers confirming that fusion had in fact taken place. This fusion trigger is where the biggest difference between the Fleischmann-Pons and NASA experiment would appear to be, with the former using purportedly the electric current to initiate fusion. As with all experimental setups, contamination and environmental factors that were not accounted for may have confounded the original researchers.
In the world of scientific inquiry, this is an important aspect to keep in mind, a point further illustrated by the wild ride that was the hype around the EmDrive. This was supposed to be a fuel-less, microwave-based thruster that was supposed to work by physics-defying means. Ultimately the measured thrust from the original experiment was refuted when all environmental influences were accounted for, resigning the concept to the dustbin of history.
An important aspect to consider here is that of intent. Many major discoveries in science began with someone looking at some data, or even a dirty Petri dish and thinking to themselves something along the lines of “Wait, that’s funny…”. No one should feel constrained to throw wild ideas out there, just because they may turn out to be misinterpreted data, or a faulty sensor.
Superconductivity
Although superconducting materials have been in use for decades, the main issue with them is that they tend to require very low temperatures in order to remain in their superconducting state. So-called ‘high-temperature‘ superconducting materials are notable for requiring temperatures that are comfortably away from absolute zero. The record holder here at atmospheric pressures is the cuprate superconductor mercury barium calcium (HGBC-CO), with a temperature requirement of only 133 K, or -140 °C.
While we don’t understand yet how superconductivity works, we have observed that increasing pressure can drastically increase the required temperature, bringing it closer to room temperature. This discovery came along with the increased focus on hydrides, following the 1935 prediction of a ‘metallic phase‘ of hydrogen by Eugene Wigner and Hillard Bell Huntington. When combined with a material such as sulfur, the required pressure of 400 GPa (~3.9 million atmosphere) to create metallic hydrogen is reduced significantly. Even more tantalizing is that metallic hydrogen is theorized to be an excellent superconductor.
Metallic hydrogen was first experimentally produced in 1996 at Lawrence Livermore National Laboratory using shockwave compression. The first claims of solid metallic hydrogen being produced came shortly after this, with many researchers having claimed to produce metallic hydrogen, though with some of the results being disputed. In 2017, rather than using the traditional diamond anvil cell (DAC) to put pressure on hydrogen, researchers used the Sandia Z Machine’s extremely strong magnetic fields to produce metallic hydrogen.
Controversy
Ranga Dias and colleagues have also published a number of papers on metallic hydrogen, including the production of it in a DAC at 495 GPa, a claim which was met with skepticism. This comes alongside the research in hydride alloys, with carbonaceous sulfur hydride (CSH) being the subject of the retracted 2020 paper. This material, when put under 267 GPa of pressure in a DAC, purportedly was superconducting at a balmy 15 °C, which would put it firmly in the realm of room-temperature superconducting materials as long as the pressure could be maintained.
Key to the claim of superconductivity lies in measuring this condition. With the speck of material trapped inside the DAC, this is not as simple as hooking up some wires and running a current through the material, and even this would not be sufficient as evidence. Instead the gold standard of measuring superconductivity is the ability to expel an applied magnetic field when the material enters the superconductive phase, yet this too is hard to accomplish with the material under test being inside the DAC.
Thus this property is inferred via the magnetic susceptibility, which requires that the magnetic noise from the environment is subtracted from the weak signal one is trying to measure. The effect is, as James Hamlin puts it, like trying to see a star when the sun is drowning out your sensors. One factor that led to the original Dias paper being retracted was due to the lack of raw data being supplied with the article. This made it impossible for colleagues to check their methodology and verify their results, and ultimately led to the do-over and this year’s paper.
Face Value
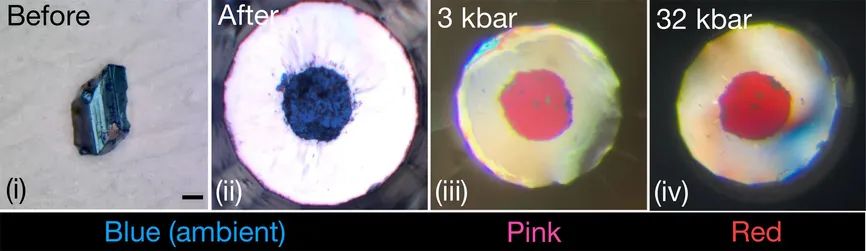
What is most interesting about this 2023 paper is perhaps that it does not merely repeat the same claims about CSH, but instead focuses on a different material, namely N-doped lutetium hydride. The claimed upper temperature limit for this material would be 20.6 ºC at a pressure of a mere 1 GPa (10 kbar, 9,900 atmospheres).
Even though it’s still early days, it shouldn’t take long for other superconductivity researchers to try their hands at replicating these results. Although the gears of science may seem to move slowly, this is mostly because of the effort required to verify, validate and repeat. The simple answer to the question of whether we’ll have room-temperature superconductivity in our homes next year is a definite ‘no’, as even if these claims about this new hydride turn out to be correct, there are still major issues to contest with, such as the pressurized environment required.
In the optimal case, these youngest results by Ranga Dias and colleagues are confirmed experimentally by independent researchers, after which the long and arduous process towards potential commercialization may conceivably commence. In the less optimal case, flaws are once again detected and this paper turns out to be merely a flash in the pan before superconductivity research returns to business as usual.
One thing remains true either way, and that is that as long as the scientific method is followed, deceptions and mistakes will be caught, as physical reality does not concern itself with what we humans would reality to be like.

![another-room-temperature-superconductivity-claim-and-questions-of-scientific-integrity-[hackaday]](https://i0.wp.com/upmytech.com/wp-content/uploads/2023/03/115203-another-room-temperature-superconductivity-claim-and-questions-of-scientific-integrity-hackaday.jpg?resize=800%2C445&ssl=1)